Posted: January 8, 2020
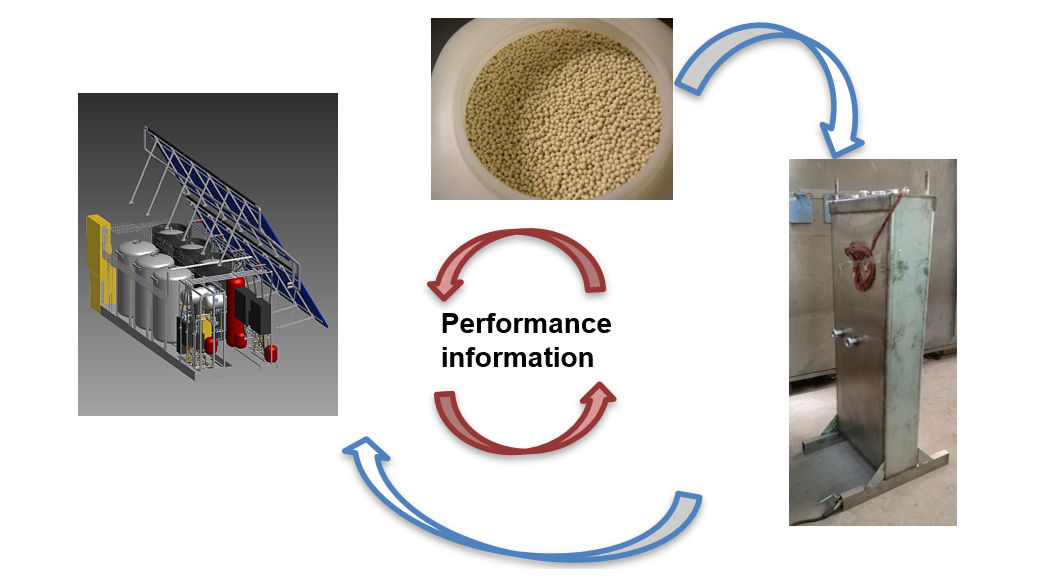
Chart: Task 58
Research into compact thermal energy storage is made difficult by the fact that the performance of storage materials depends significantly on their intended purpose. For example, storage tanks that are integrated into space heating systems are made of materials different from those used to make tanks for process heat loops generating steam. With this in mind, the members of the international research platform Material and Component Development for Thermal Energy Storage have taken a two-pronged approach to finding new storage solutions by looking for new and advanced materials and examining component and system design at the same time (see the image above). Wim van Helden, who co-chairs the platform called IEA SHC Task 58, explained the researchers’ approach and key findings during a webinar held by the IEA Solar Heating and Cooling programme’s Solar Academy on 27 November. A recording of the webinar and all presentations given at the event are available here.
According to van Helden, the approach used by the Compact Thermal Energy Storage (CTES) group is “research in cycles”. He said: “We may start with the material, for example, the zeolite beads in the photo above. They have certain properties that determine what the chemical reaction will look like. Related to this, we begin defining a reactor, seen at the bottom right, and its performance will again determine the design of the entire system [photo on left]. “ The researchers need to go through this cycle several times to fully optimise a system for a particular application.
However, van Helden underlined that the researchers also have to pass the cycle in reverse. In this case, the first step is to estimate system performance by using a specific reactor and material. “Afterwards, we go back to the reactor and analyse how we can improve the charge and discharge components for the material to maximise its performance.”
The Material and Component Development for Thermal Energy Storage group consists of around 60 materials and application experts based in 13 different countries. This interdisciplinary team allows for efficient research on the integration of CTES into certain applications. Each researcher is working on both common objectives and one of over 40 independent projects, all of which examine different aspects of CTES.
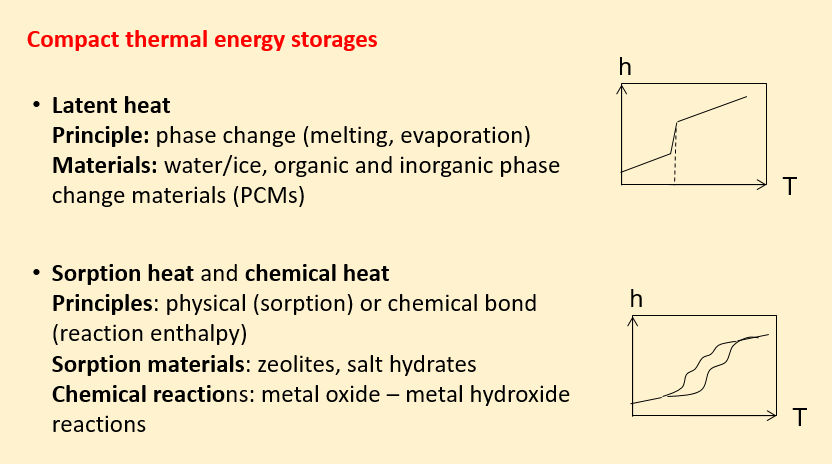
Compact thermal energy storage retains heat in different ways: as latent heat through melting or evaporation, physically via sorption or chemically by means of reaction enthalpy.
Source: Task 58
New thermochemical materials show ultra-high performance
The three-year international research platform will end at the beginning of 2020, and the researchers used the opportunity offered by the webinar to present the key findings of their collaborative work. The following chart shows some of the results related to sorption / chemical heat storage. The group is now creating a soon-to-be-available overview of the characteristics and properties of existing and recently discovered thermochemical materials.
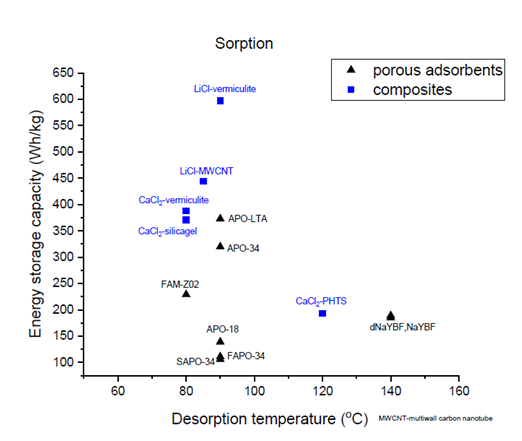
Characteristics of known and new sorption materials
Source: National Institute of Chemistry, Slovenia
The materials experts within the group have developed special sorption materials with a higher energy storage capacity (blue squares) than that of porous sorption materials (black triangles). The above graph, created by the Slovenian National Institute of Chemistry, shows the energy storage capacity (in Wh/kg) plotted over the temperature at which the material has been charged (x-axis). The latter is also called the desorption temperature.
At the top of the list of high-capacity materials are vermiculite minerals impregnated with a lithium-chloride solution (LiCl-vermiculite). This combination stores up to 600 Wh/kg, which is nearly double the energy capacity of the best-performing, pure sorption material, APO-LTA, a Linde Type A aluminium phosphate. LiCl is widely available, but the addition of lithium makes it relatively expensive and it has yet to be tested in a reactor.
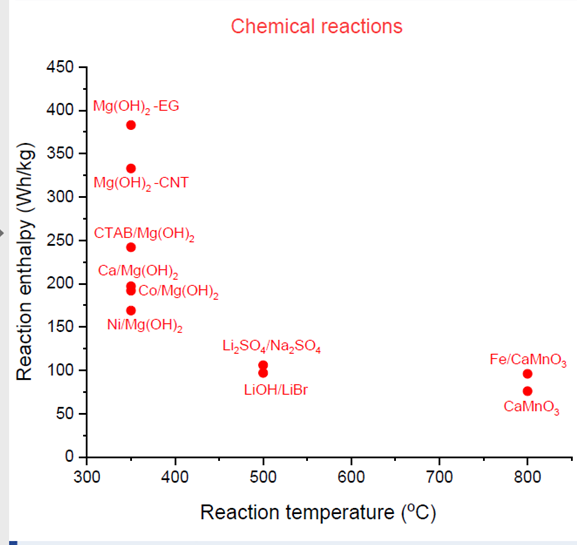
Characteristics of new chemical reactions
Source: National Institute of Chemistry, Slovenia
In addition, much research has been conducted on what two materials could be combined to trigger a thermochemical reaction that will store thermal energy for process heat applications from 300 °C to 800 °C. The reaction enthalpy and the reaction temperature of some of them are depicted in the chart above. The thermal champion in this particular case is magnesium hydroxide mixed into expanded graphite, known as Mg(OH)2-EG. Its enthalpy is between 350 Wh/kg and 400 Wh/kg at a temperature of about 370 °C. A prototype reactor for magnesium hydroxide has already been developed by the Vienna University of Technology.
Organisations mentioned in this article: